The location of the new campus will be just over the Yokneam municipal lines in Kiryat Tivon.
Globes reports that Boston Scientific is transferring Galil Medical activity to Ireland
Just as NVidia doubles down on Israel (and the Yokneam area in particular), Boston Scientific has decided to move the Galil Medical activity that it acquired to Ireland.
Nvidia Israel links data centers across continents
On Friday, NVidia announced a technological breakthrough by their Yokneam R&D center that will allow data centers in geographically distant locations to operate as if they were in one place, effectively creating “AI factories” on a massive scale and significantly increasing the maximum computing power available to the industry.
Wearable Devices Secures U.S. Patent for Groundbreaking Neural Interface Technology
The patent protects Wearable Devices’ proprietary neural interface technology that enables gesture-based control and real-world physical measurements directly from the wrist.
NVIDIA plans massive tech campus in Israel, boosting AI innovation
Yokneam’s largest Hi-Tech company, NVIDIA, is looking for 29 acres to build a new R&D hub that will expand its presence. This major expansion of what began as Mellanox will continue to transform the entire AI industry.
Israeli tech sector raises $9b in funding in first half of 2025
According to a mid-year analysis by Startup Nation Central, roughly $9.3 billion was invested in Israeli hi-tech since the beginning of the year, a 54% jump compared to the second half of 2024.
Cytora Reports Phase 1 Data of Stem Cell Treatment for Multiple System Atrophy
Clinical data of Cytora's oral mucosa stem cells treatment shown to be safe and may be efficient as a disease modifying therapy in moderate stages of Multiple System Atrophy
Nvidia Israel to benefit from huge Saudi deal
Nvidia's development and production centers in Israel will benefit from the huge deal and will be able to sell their wares in Saudi Arabia, even though it has decided not to recognize Israel.
No End In Sight For Hostages - Israel News Insights - April 21, 2025 (Day 562)
On Friday, the Prime Minister made a dramatic announcement that he would be making an important — albeit recorded — press conference on Saturday night, thus raising the hopes of the hostages’ families that he would finally be ending the war and returning all of their loved ones. Their hopes were quickly dashed the next night...
This week we have a number of different articles that do not necessarily reflect the opinions of all of our editors - so you may see a number of different focuses. These include:
Editorial: Are We Being “Two-Faced” When We Tell the World Israel Is Truly Free?
The Cost of Protecting Israel’s First Family, and the Efforts to Cover it Up
The Road to Pravda - Rock Throwing Yutes
Harari’s Playbook for Dictatorship: A Chilling Parallel to Netanyahu’s Actions
If you disagree with some of these - that’s a good sign and all the more reason to forward the newsletter to friends and relatives!
Do Not Pass Them Over - Israel News Insights - April 14, 2025 (Day 555)
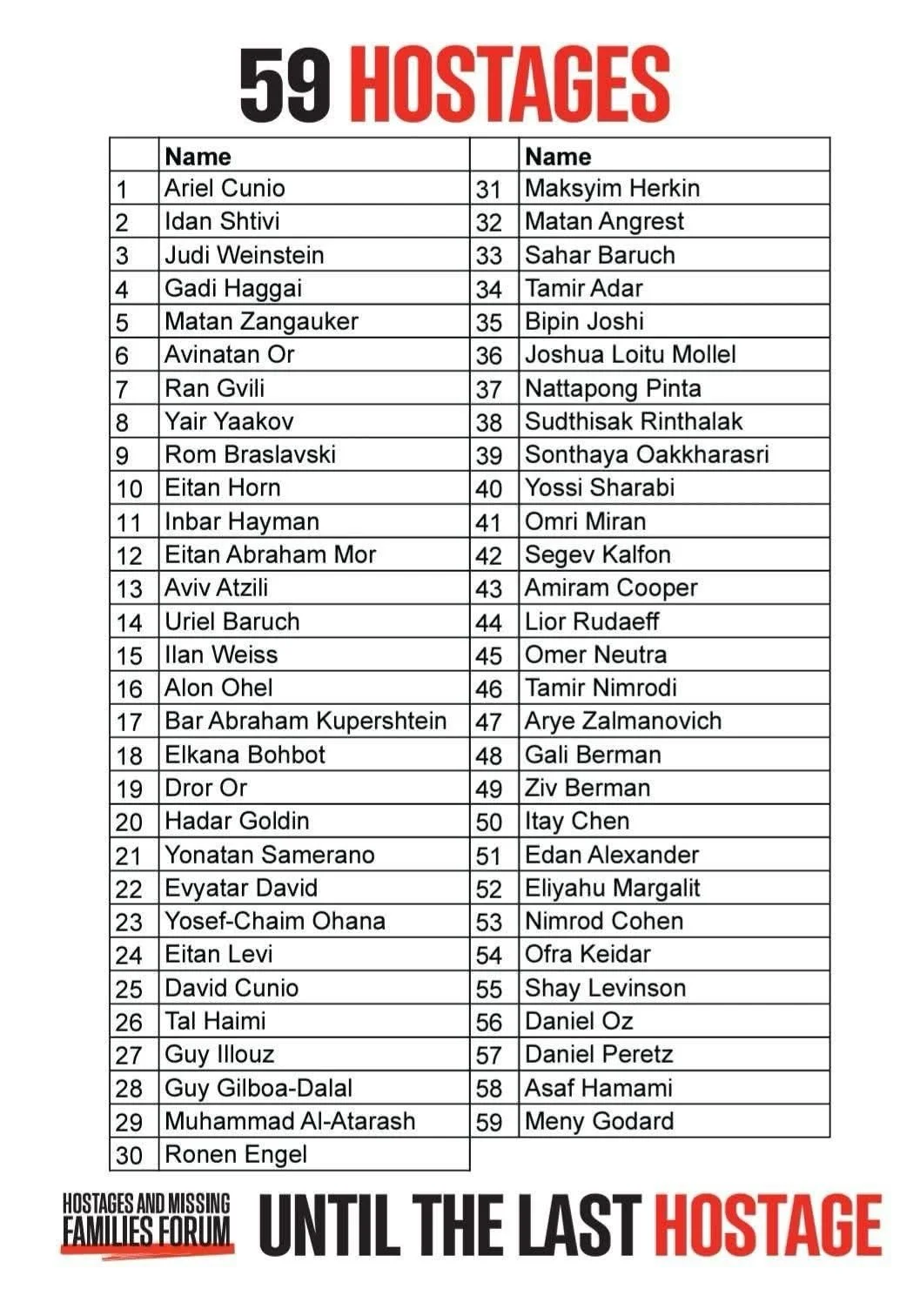
This Passover has been as bittersweet a holiday as it’s ever been (or at least since the Holocaust). Having family together and celebrating freedom is always sweet, but this year it almost feels like we are still waiting for sea to part because 59 of our people are still being held in conditions worse than the slaves of Egypt.
We know that you are busy and preoccupied with Passover week and for most of you, who live outside of Israel, celebrating the 2nd Seder. So we’ll keep this week’s newsletter short. And we ask you to do at least one thing each day of Passover week to keep the fate of the remaining 59 hostages in the public eye. Ideas… Tie yellow ribbons in public places, call or write a local politician or news source, call for President Trump to step in where Prime Minister fails to act (sounds terrible - but Bibi is no Moses).